Pregnancy Complications
Infant- and Family-Centered Developmental Care
Respiratory Infections
Neonatal Eye Health
Hygiene
Rare Diseases

IMPROVE PRETERM is a four-year European research project working to improve the lifelong health and development of children and adults born very preterm – before 32 weeks of pregnancy.
Thanks to advances in neonatal care, more very preterm babies survive today than ever before. But survival is only the beginning. Many children and adults born very preterm face long-term challenges affecting learning, physical health, mental health, and quality of life. Families and healthcare providers often lack clear, evidence-based guidance on what care and follow-up work best over time.
IMPROVE PRETERM brings together researchers, clinicians, families, and advocates from across Europe to close these gaps. The project focuses on generating better evidence, developing practical tools, and strengthening follow-up care – so children born very preterm can not only survive, but thrive.

Very preterm birth is a major public health issue worldwide. Children born too soon are at higher risk of developmental delays, cerebral palsy, respiratory illness, learning difficulties, and mental health conditions that can persist into adulthood.
While neonatal care has improved survival, long-term outcomes have not improved at the same pace. One key reason is the lack of strong, consistent evidence about which treatments, follow-up programs, and preventive strategies work best across the life course.
Research in this area faces real challenges:
IMPROVE PRETERM addresses these gaps by combining large-scale data, modern research methods, and family perspectives to generate clearer, more useful evidence for real-world care.

IMPROVE PRETERM is a large international collaboration involving 20 partner organizations from 13 countries. The project runs from January 2025 to December 2028 and is funded by the European Union’s Horizon Europe Research and Innovation program.
The work is organized into six interconnected workstreams, covering:
This structure ensures that scientific research, lived experience, data infrastructure, and communication all work together toward the same goal: better lifelong outcomes for people born very preterm.
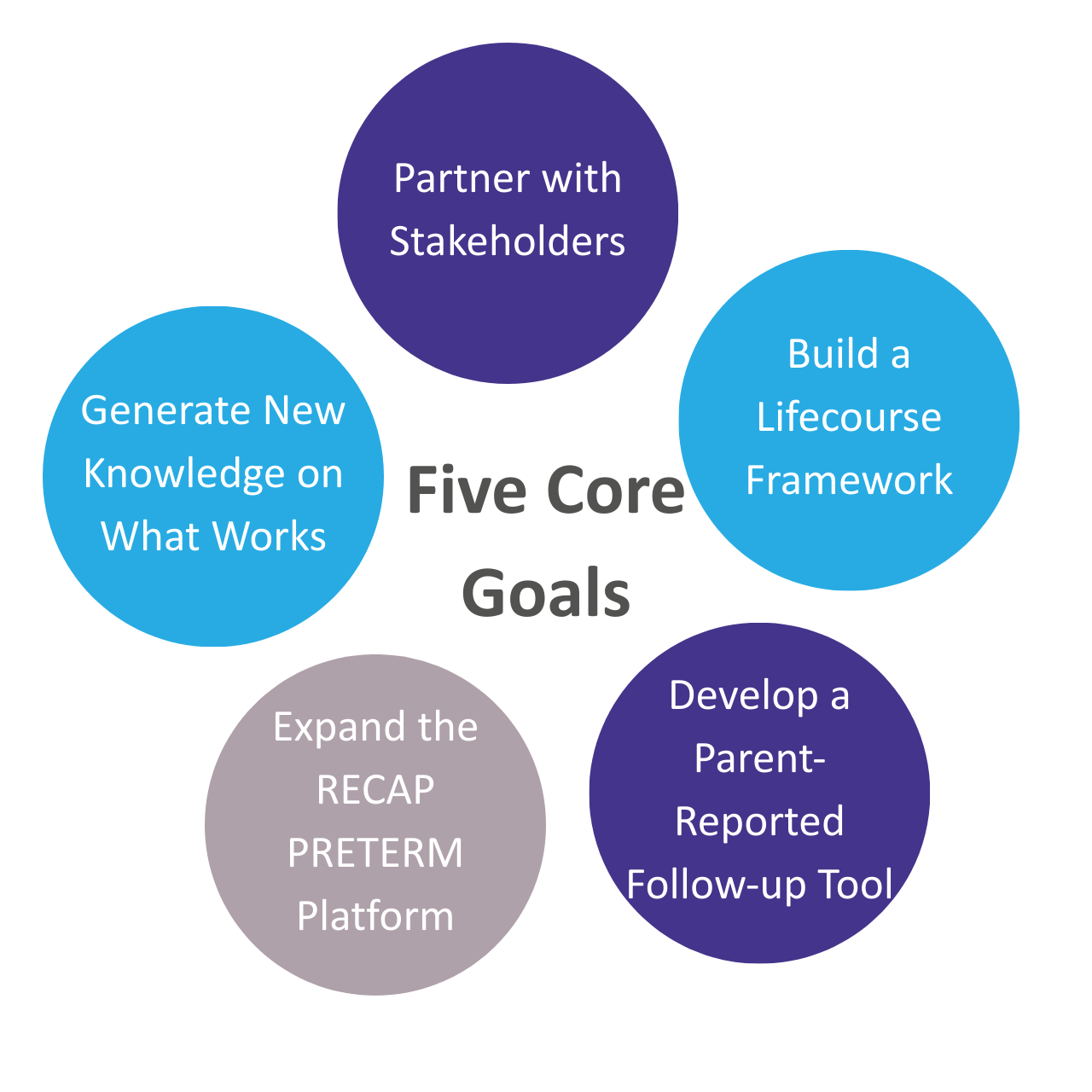
IMPROVE PRETERM has five core objectives:
Together, these aims support more informed care, fairer follow-up systems, and better long-term health for very preterm individuals.

GFCNI leads stakeholder and family involvement, communication, and dissemination within IMPROVE PRETERM.
GFCNI ensures that:
By bridging science and lived experience, GFCNI helps ensure that IMPROVE PRETERM delivers outcomes that are meaningful, ethical, and relevant to real life.
In IMPROVE PRETERM, GFCNI leads stakeholder and family involvement, communication, and dissemination activities. GFCNI ensures that families’ lived experiences help shape research priorities, tools, and outcomes, and that project findings are shared in clear, accessible language with families, healthcare professionals, and policymakers. By connecting science with real-life experiences, GFCNI helps ensure that research leads to meaningful, family-centered improvements in care.
For further information, please contact: research(at)gfcni.org
IMPROVE PRETERM brings together a multidisciplinary consortium of leading institutions, each contributing unique expertise:
INSERM (France) – Project coordination; expertise in epidemiology, biostatistics, and comparative effectiveness research
University of Oulu (Finland) – Research on perinatal and neonatal interventions using Nordic register data
University of Leicester (UK) – Development and validation of the PARCA-5/7 parent-reported follow-up tool
Norwegian University of Science and Technology – NTNU (Norway) – Research on follow-up and early intervention programs
University Hospital Würzburg (Germany) – Clinical trials and developmental pediatrics; German Neonatal Network data
Institute of Public Health, University of Porto – ISPUP (Portugal) – Public health research and qualitative studies
University of Oxford (UK) – Health economics and policy analysis
Karolinska Institutet (Sweden) – Pediatric neurology, psychology, and Swedish neonatal register data
University of Copenhagen (Denmark) – Life-course epidemiology and register-based research
University Hospital Antwerp – UZA (Belgium) – Neonatal care, follow-up, and family-centered care
Eugenio Medea Scientific Institute – MEDEA (Italy) – Follow-up research, ethics, and qualitative studies
University of Tartu (Estonia) – Neonatology, child psychology, and cohort data
Varha Wellbeing Services County (Finland) – Neonatal follow-up and regional healthcare data
University of Warwick (UK) – Research on adult outcomes after very preterm birth
Poznan University of Medical Sciences – PUMS (Poland) – Neonatal care, clinical trials, and infectious disease research
INESC TEC (Portugal) – Software engineering, artificial intelligence, and data analysis tools
Epigeny (France) – Secure, privacy-preserving data platforms and open-source research software
INSERM Transfert (France) – Project management and innovation support
University of Zurich (Switzerland) – Associated partner contributing neonatal and follow-up expertise
Together, this team combines scientific excellence, clinical expertise, technical innovation, and lived experience to improve care for very preterm individuals across Europe.
The Parent & Patient Advisory Board (PPAB) brings together parents of children born very preterm and adults who were themselves born very preterm.
The PPAB plays a central role in IMPROVE PRETERM by providing lived-experience perspectives throughout the project. Members advise researchers on which outcomes matter most to families, help shape research tools and communication, and offer feedback to ensure that project activities remain respectful, relevant, and grounded in real-life needs. Through the PPAB, families are partners in research – not just subjects of it.
Members of the PPAB are:
Vilni Verner Holst Bloch, “Prematurforeningen” (Norway)
Elzbieta Brzozowska, “Koalicja dla wcześniaka Fundacja” (Poland)
Adriana Guerreiro, “Nascer Prematuro” (Portugal)
Dina Hediger, “Frühchen & Neokinder Schweiz” (Switzerland)
Maria Hitzschke, Bundesverband “Das frühgeborene Kind e.V.” (Germany)
Lauren Ingledow, “Adult Preemie Advocacy Network” (United Kingdom)
Kristel Kuuk, “MTÜ Enneaegsed lapsed” (Estonia)
IMPROVE PRETERM has its own project website with in-depth information on the research, partners, and results as the project progresses. If you’d like to explore the project in more detail, you can visit the official IMPROVE PRETERM website.
© 2026 GFCNI. All Rights Reserved.