Pregnancy Complications
Infant- and Family-Centered Developmental Care
Respiratory Infections
Neonatal Eye Health
Hygiene
Rare Diseases

ArtPlac is a preclinical research project developing a novel artificial placenta to support critically ill newborns in intensive care. The technology is designed to assist both the lungs and kidneys, enabling gentler, less invasive monitoring and life support.
The ArtPlac project is an innovative international initiative funded by the European Innovation Council and SMEs Executive Agency (EISMEA). Launched in April 2023, the project runs for four years and brings together 11 leading research and advocacy institutions from Germany, the Netherlands, Sweden, Ireland, Portugal, and Canada.
ArtPlac has the potential to save newborn lives worldwide each year, significantly reducing the risks and medical burden these infants face.
Each year, approximately two million newborns die globally, many due to organ failure, particularly affecting the lungs and kidneys - a frequent complication among preterm infants [1].
Historically, medical devices and interventions used for newborns were adapted from adult care technologies, simply scaled down in size. While these approaches aimed to address life-threatening conditions, they often proved too invasive for fragile newborns, sometimes causing additional harm rather than promoting recovery [2-5].
The ArtPlac project seeks to develop a less invasive treatmentmethod forpreterm and critically ill newborns, preventing complications in vital organs.
Before birth, the placenta performs many critical bodily functions for the baby, including supporting lung and kidney function and providing nutrition. Since the placenta cannot be reconnected after birth, ArtPlac introduces a groundbreaking artificial alternative.
The ArtPlac device connects to the baby’s umbilical cord and supports both lung and kidney function - mimicking the natural role of the placenta in utero. Powered entirely by the infant’s own heartbeat, the system allows for the collection of bodily data in a far less invasive way.
This innovative device not only delivers vital health information to clinicians and supports organ function, but also creates a gentler, more natural environment that fosters healing and development.
Importantly, ArtPlac enables closer physical contact between babies and their families, overcoming the barriers posed by conventional medical equipment. This advancement supports infant- and family-centered developmental care (IFCDC), strengthening the emotional and physical bonds critical for early development.
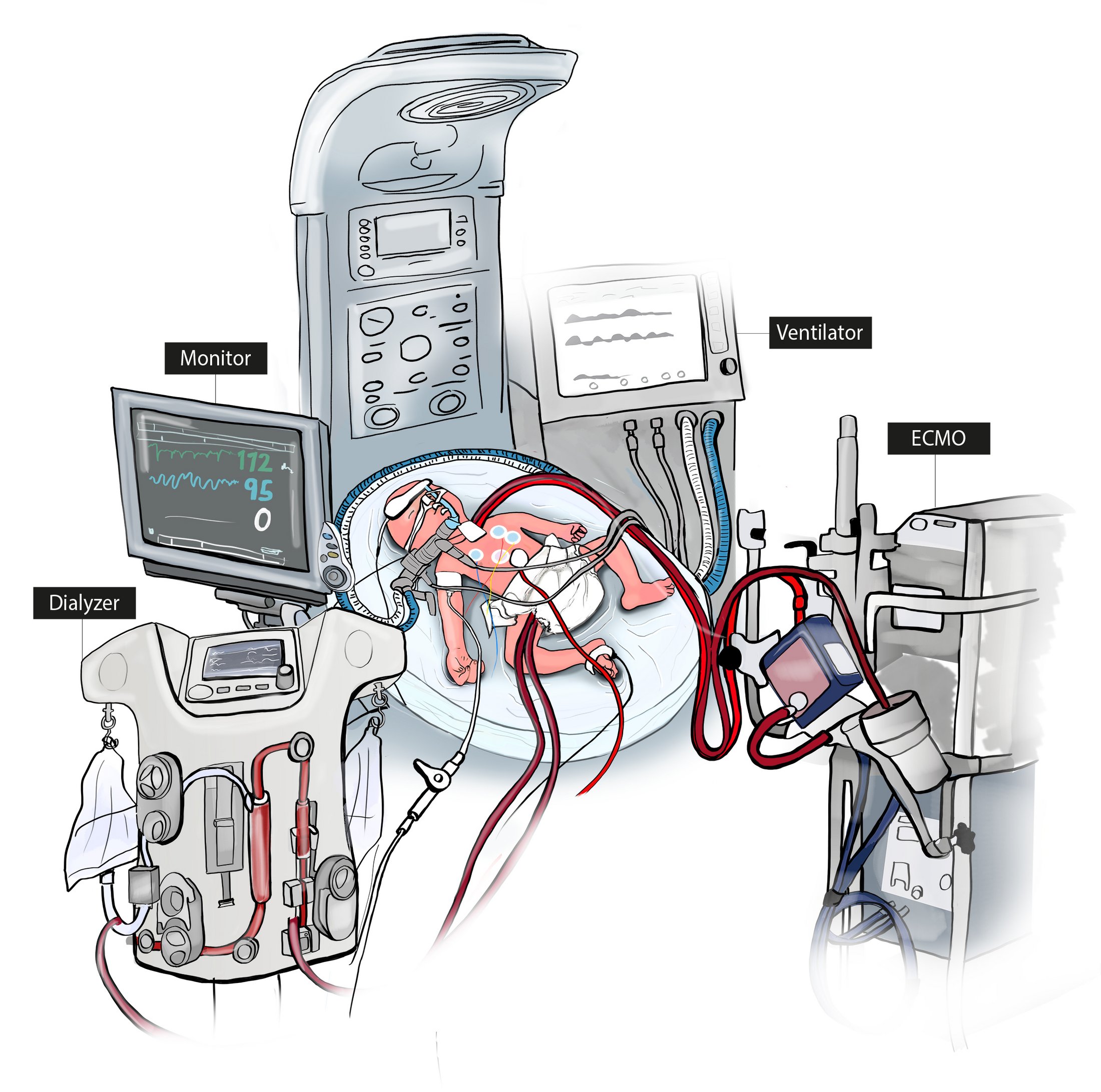
State-of-the-Art Intensive Care
Figure 1A illustrates a typical current treatment setup for critically ill newborns, showing the invasive state-of-the-art setup with respirator, four surgically placed lines, attached extracorporeal lung assist and dialyzer with paralyzed newborn.
The Research Project's Approach: ArtPlac
Figure 1B and 1C present the envisioned future using the ArtPlac device - a novel technology in development. This minimally invasive device connects to the baby’s umbilical cord and provides lung and kidney support while also monitoring vital functions in real time. By minimizing cables, the artificial placenta enables parents to interact more closely with their child, supporting infant and family-centred developmental care, which leads to improved outcomes for the whole family.
To ensure that the interests and needs of patients, parents, and caregivers are considered in the scientific process, it is important to include input from representatives during every stage of the project. Ongoing feedback from families ensures the technology truly fits real-life hospital settings and families' needs during neonatal intensive care, supporting infant- and family-centered developmental care (IFCDC). Their firsthand experiences help design a solution that allows parents to stay close to their newborns while reducing stress and medical burden.
To facilitate this exchange, GFCNI established a Patient Advisory Board (PAB) made up of five members from diverse geographical and professional backgrounds. The five PAB members have joined the ArtPlac consortium in late 2023 and have participated in multiple PAB meetings since then.
Some meetings involved the entire project consortium, providing patient and parent representatives with general updates on progress, including current challenges, findings, and achievements. Others took place in smaller groups to explore specific important aspects in greater depth.
Between scheduled PAB meetings, the patient and parent representatives have continued contributing to the project by offering valuable feedback on ethical considerations, prototypes, illustrations, and the role of skin-to-skin care — consistently emphasizing the importance of inclusive design and their commitment to being involved in every stage of development.
The meaningful input of the PAB is helping shape ArtPlac into a life-changing medical device that supports both clinical outcomes and family connection — a core mission shared by the entire ArtPlac team.
PAB meetings, involving the ArtPlac consortium and the five dedicated PAB members:
The international project brings together 11 leading research and advocacy institutions from Germany, the Netherlands, Sweden, Ireland, Portugal and Canada, being supported with valuable input by an international Patient Advisory Board (PAB):
Klinikum Nürnberg leads the consortium, which includes:
Representative of the EU’s diversity, the ArtPlac PAB includes five individuals from across Europe, who have widely differing professional backgrounds:
Under the lead of GFCNI, the PAB was established and is subsequently managed throughout the project. This includes the organization of meetings and facilitation of exchanges between the project consortium and the patient and parent representatives.
GFCNI not only manages the involvement of the PAB, but also represents all parent’s and patient’s voices in regular Steering Committee Meetings, ensuring that the device reflects the realities of hospital life and the emotional, logistical, and practical needs of families.
Howson, C. P. et al. Reprod Health 10, S1 (2013).
Heron, M. et al. Natl Vital Stat Rep 57, 1–134 (2009).
Stoll, B. J. et al. Pediatrics 126, 443–456 (2010).
Koyner, J. L. Et al. Blood Purif 29, 52–68 (2010).

Acknowledgment of EU Funding: This project has received funding from the European Union’s Horizon Europe research and innovation program under Grant Agreement No. 101099596.
Funded by the European Union. The views and opinions expressed are those of the author(s) and do not necessarily reflect those of the European Union or the Horizon Europe research and innovation program. Neither the European Union nor the granting authority is responsible for any use that may be made of the information contained herein.
© 2026 GFCNI. All Rights Reserved.