
The EU-funded RECAP preterm project aimed to improve the health, development, and quality of life of children and adults born very preterm (VP) or with a very low birth weight (VLBW):
Very Preterm (VP): Born before 32 weeks of gestation
Very Low Birth Weight (VLBW): Weighing less than 1,500 grams at birth
The innovative goal of RECAP preterm was to bridge the gap between data collection and data sharing. The project established a digital platform to harmonize and utilize data from European cohort studies involving individuals born preterm, as well as Nordic registry data.
By combining and analyzing these diverse datasets, the project aimed to improve understanding, diagnosis, and evidence-based, personalized prevention of mental and physical health disorders associated with preterm birth. A particular focus was placed on analyzing the long-term effects of medical treatments, including the use of off-label medications, by linking data from adult cohorts with data from preterm infants.
To support long-term follow-up, the project also developed mHealth applications (mobile health tools) to encourage participants to continuously contribute health data.
mHealth refers to the use of digital technologies to collect health information, deliver healthcare content, monitor vital signs, and enable telemedicine.
The RECAP preterm project:
Created a sustainable data platform that integrates national and European cohorts of VP/VLBW children and adults, optimizing the use of population-based data for research, healthcare innovation, and policy development
Developed hypothesis-driven research on the health status and medical care of VP/VLBW individuals, leveraging the large sample sizes and geographic and temporal diversity of combined cohorts
Engaged with key stakeholders to disseminate findings and support evidence-based care and policy making
Stakeholders included obstetricians, neonatologists, pediatricians, psychologists, psychiatrists, educators, scientists, economists, healthcare providers, policy planners, insurers, and patient and parent groups

The RECAP preterm Consortium
RECAP preterm brought together a wide range of European child-to-adult cohorts and a group of highly experienced organizations across multiple disciplines. The consortium included experts in:
Life course epidemiology
Clinical methodology
Neonatology and pediatrics
Epigenomics and mental health
Psychology, economics, and health policy
eHealth and mHealth applications
e-learning technologies
This diverse expertise enabled a comprehensive, multidisciplinary approach to studying the long-term health outcomes of individuals born very preterm (VP) or with a very low birth weight (VLBW).
GFCNI (formerly EFCNI) served as the patient voice within the RECAP preterm project and led the work package on dissemination and communication.
GFCNI was responsible for:
Coordinating all activities related to dialogue, dissemination, and long-term sustainability
Engaging with the scientific community, clinicians, healthcare professionals, patient organizations, policymakers, and the general public at the national, European, and international levels
Enhancing the visibility, societal relevance, and health impact of the RECAP preterm project
Placing a strong focus on parent involvement to ensure that research and policy efforts responded to real-world needs and user-driven questions
This engagement was essential to align the project’s goals with patient-centered care and to inform evidence-based policy.
To learn more about GFCNI’s role in RECAP preterm, patient involvement, and our broader contributions to research projects, watch the module here:
The RECAP preterm e-learning course offers valuable insights into:
Very preterm (VP) cohort studies and principles of collaborative research
New research findings on children and adults born very preterm, based on pooled cohort data
Access the e-learning course to explore the full capabilities of the RECAP preterm platform and learn more about the project’s methodology and impact:
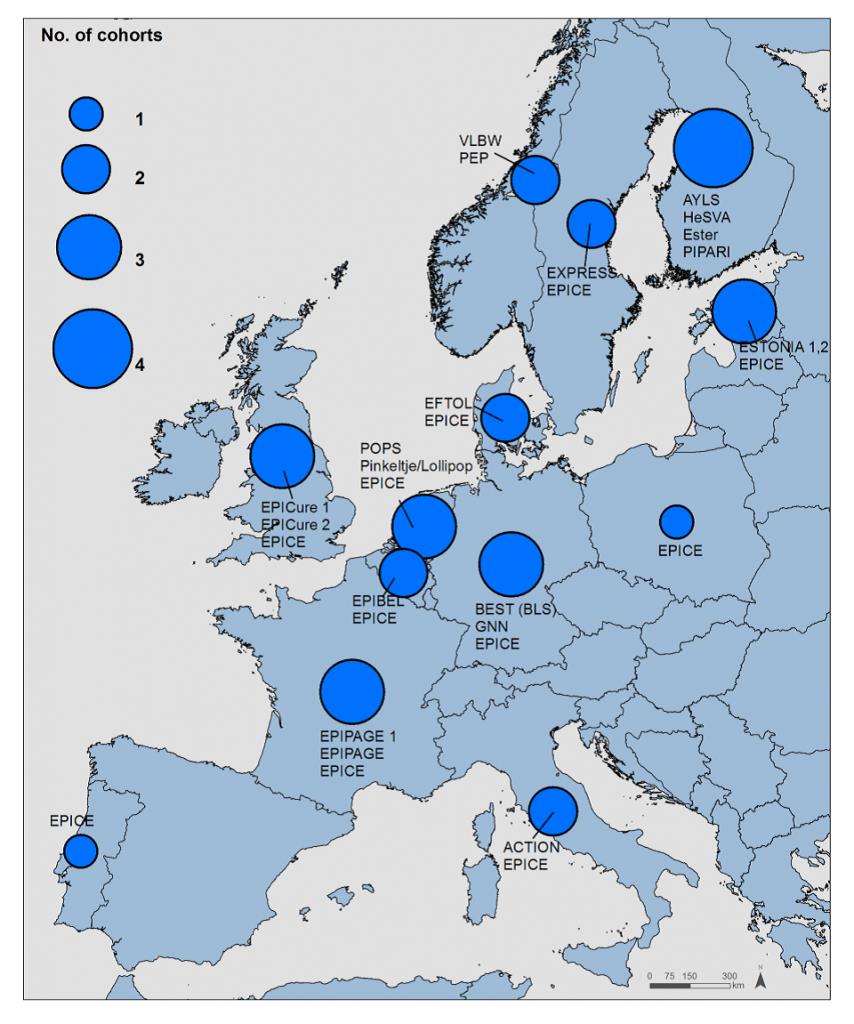
In a year-long series, RECAP preterm showcased several of its contributing European cohort studies, highlighting the diversity and depth of research into outcomes of preterm birth. Each article focuses on one or more cohorts involved in the project.
Featured Cohorts:
Part 1: The ESTONIA I & II cohorts
Part 2: The EPICure studies in the United Kingdom and Ireland
Part 3: The Project Extreme Prematurity (PEP) from Norway
Part 4: The NTNU Low Birth Weight Life study from Norway
Part 5: The Bavarian Longitudinal Study (BLS) / Bayerische Entwicklungsstudie (BEST) from Germany
Part 6: The POPS (Project On Preterm and Small for Gestational Age Infants) cohort from the Netherlands
Part 7: The EPIBEL cohort (Extremely Preterm Infants in Belgium)
To gain a comprehensive understanding of the effects of medical interventions, both short-term and long-term follow-up studies are essential. With this in mind, the RECAP preterm consortium developed the RECAP Preterm Cohort Platform – a sustainable, geographically diverse, and multidisciplinary database of national and European cohorts of individuals born very preterm (VPT) or with a very low birth weight (VLBW).
This platform helps reduce ethical, administrative, and technical barriers to research collaboration while creating positive incentives for engagement among research teams across the globe. It enables timely responses to emerging questions related to best practices in care, health outcomes, and social policy for individuals born preterm or with low birth weight.
By bridging the gap between data collection and data sharing, the platform offers long-term value that extends beyond the original RECAP preterm project timeline.
As new cohorts are added and existing data is updated, the RECAP Preterm Cohort Platform has the potential to evolve into a permanent European infrastructure supporting healthcare innovation and scientific research.
One example of this evolution is the LIFT-UP Preterm project, which builds upon the RECAP platform with innovative methods for long-term follow-up of very preterm patients involved in randomized controlled trials. It also uses observational population-based cohort data to enhance analyses and strengthen findings.
LIFT-UP Preterm contributes to the expansion and application of the RECAP Preterm Cohort Platform by:
Developing a data entry tool integrated into the platform, based on the open-source PARCA-R questionnaire – a parent-completed tool used to assess cognitive and language development in children at 24 months of age
Conducting a proof-of-concept study using data from the TREOCAPA trial, a multi-center, pan-European randomized controlled trial
Explore the poster to see an overview of the project’s aims:
© 2025 GFCNI. All Rights Reserved.