
IFT-UP Preterm is an innovative research project led by INSERM (the French National Institute of Health and Medical Research). The project aims to improve health outcomes for very preterm populations by using advanced research methods and tools.
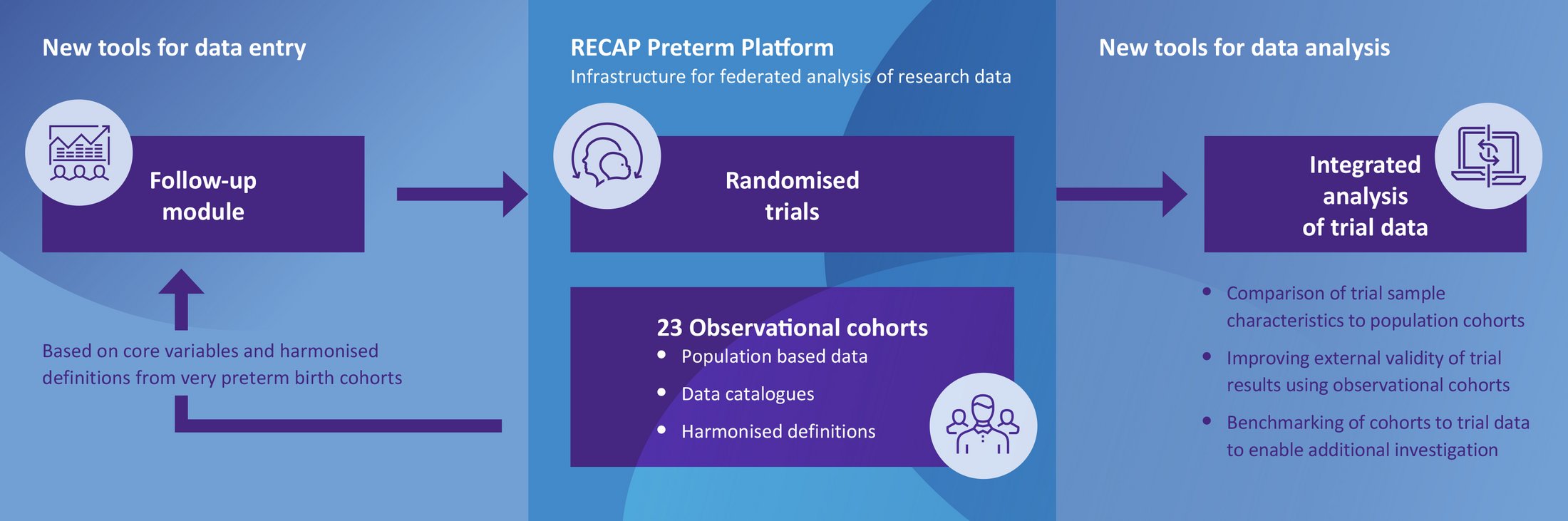
LIFT-UP Preterm leverages the existing RECAP Preterm Cohort Platform, a European research database designed to enhance the health, development, and quality of life of individuals born very preterm or with a very low birth weight.
For more details on RECAP Preterm, visit GFCNI’s RECAP Preterm project page.
The project brings together 8 academic partners and 24 hospitals from 13 European countries. LIFT-UP Preterm will run for a planned duration of four years.
To ensure scientific rigor and sustainable progress in the life sciences, researchers often rely on Randomized Controlled Trials (RCTs) to compare the effectiveness of drugs, medical techniques, or other treatments. These trials generate essential knowledge that helps refine healthcare practices and interventions.
However, due to the complex nature of neonatal care and the high costs of conducting RCTs, such trials are less common in the neonatal population and require dedicated attention.
Conducting long-term follow-up investigations is crucial for gaining a comprehensive understanding of medical interventions. These follow-ups help avoid overlooking potential long-term consequences of treatments initially evaluated in RCTs.
The main goal of the LIFT-UP Preterm project is to improve health outcomes for individuals born very preterm by advancing the use of Randomized Controlled Trials (RCTs) in this vulnerable population.
One of the project’s key objectives is to develop an integrated data entry tool that allows parents to report on their child’s cognitive development through a parent-completed questionnaire. By linking this new data to the RECAP Preterm Cohort Platform, researchers can analyze the follow-up results in conjunction with existing baseline data. This approach helps uncover the long-term effects of early medical interventions.
Another important focus of the project is to improve data comparability across individual data sets and cohorts within the RECAP Preterm platform, enabling more consistent and reliable research outcomes.
A central component of LIFT-UP Preterm is the TREOCAPA-long-term study (TREOCAPA-LT) — a proof-of-concept study conducted under the umbrella of the project.
The original TREOCAPA trial investigates the use of preventive paracetamol in babies born preterm, administered within the first five days of life to help close the patent ductus arteriosus (PDA). The trial aims to reduce mortality and severe complications related to preterm birth during the neonatal period.
The ductus arteriosus is a blood vessel that connects the aorta (the body’s main artery) to the pulmonary artery (which carries blood to the lungs for oxygenation) while the baby is still in the womb. In most newborns, this vessel closes shortly after birth. However, in many preterm infants, it remains open, which can lead to complications affecting the lungs, brain, or gastrointestinal system.
For more details on the original trial, visit GFCNI’s TREOCAPA project page.
Even early medical interventions can have long-lasting impacts on a person’s health. That’s why it's essential to take a long-term perspective. The TREOCAPA-LT study addresses this need by extending the observation period beyond the initial trial.
The main goal of TREOCAPA-LT is to follow up with the participants of the original TREOCAPA trial to evaluate the safety and effectiveness of the treatment when the children reach two years of age. A special emphasis is placed on identifying potential neurological disorders or cognitive development issues.
Parents or caregivers of children who took part in the original trial are asked to complete a questionnaire once their child reaches 24 months. This questionnaire helps determine any long-term health effects of early paracetamol treatment, with careful consideration of the child’s gestational age at birth.
Integrating this new dataset into the RECAP Preterm Cohort Platform adds valuable longitudinal data. It offers a more comprehensive view of the study population by extending the observation window across both TREOCAPA and TREOCAPA-LT, supporting the sustainable use of collected research data.
GFCNI (formerly EFCNI) has played a key role in both the RECAP Preterm project and the original TREOCAPA trial, and continues its involvement in the TREOCAPA-LT study. As a representative of patients and parents, GFCNI ensures that families' voices remain central throughout the research process.
GFCNI contributes by raising awareness about the project and by developing educational materials that help communicate the importance and outcomes of the studies to a broader audience. These efforts promote knowledge sharing and enhance public understanding of innovative research in neonatal care and preterm health.
Planned materials may include:
A fact sheet on the importance of follow-up studies in life sciences
Explanatory materials for parents, highlighting project progress and findings
A Randomized Controlled Trial is a study designed to measure the effectiveness of a specific intervention. This could include testing a new medical treatment, drug, or procedure compared to an existing one. Participants are randomly assigned to either a treatment group or a control group. In most RCTs, neither the participants nor the researchers know who is in which group, which helps eliminate bias and ensures scientific accuracy.
RCTs are considered the gold standard in clinical research, as they minimize the impact of external factors. However, they require significant time and financial resources to conduct.
A cohort refers to a defined group of people who participate in a research study. This group typically shares a common characteristic, such as age, location, or a specific life event. In TREOCAPA-LT, the cohort consists of all the participating children who were involved in the study.
A proof-of-concept study is designed to demonstrate the feasibility and value of a new idea, approach, or method. In this case, TREOCAPA-LT serves as a proof-of-concept by showcasing how existing data and research infrastructure from the original TREOCAPA trial can be used to conduct long-term follow-up research. This efficient use of resources supports sustainable and impactful scientific practices.
For additional information on clinical trials in newborns and clear explanations of related terms, please download our factsheet below.
The TREOCAPA-LT study receives funding through the MESSIDORE program (Méthodologie des ESSais cliniques Innovants, Dispositifs, Outils et Recherches Exploitant les données de santé et biobanques – Innovative clinical trials methodology, devices, tools, and research using health data and biobanks).
This program is part of the 2022 Strategic Program for Collaborative Health Research, coordinated by INSERM (the French National Institute of Health and Medical Research).
Reference: Inserm-MESSIDORE N°74.
© 2025 GFCNI. All Rights Reserved.