
The TREOCAPA clinical trial (Prophylactic treatment of the ductus arteriosus in preterm infants by acetaminophen) focuses on the preventive treatment of patent ductus arteriosus (PDA), a common complication in very preterm infants. The study explores the effectiveness of acetaminophen (paracetamol) as a prophylactic therapy to prevent PDA.
The trial is being conducted across approximately 65 Neonatal Intensive Care Units (NICUs) in 17 European countries. It includes preterm infants born between 23 and 28 weeks of gestation. The project is sponsored by INSERM, a leading French biomedical research institute, and led by Nantes University Hospital in France.
TREOCAPA is part of the Proof of Viability phase of the conect4children (c4c) initiative, which aims to establish a pan-European pediatric clinical trial network to improve research and care for children across Europe.
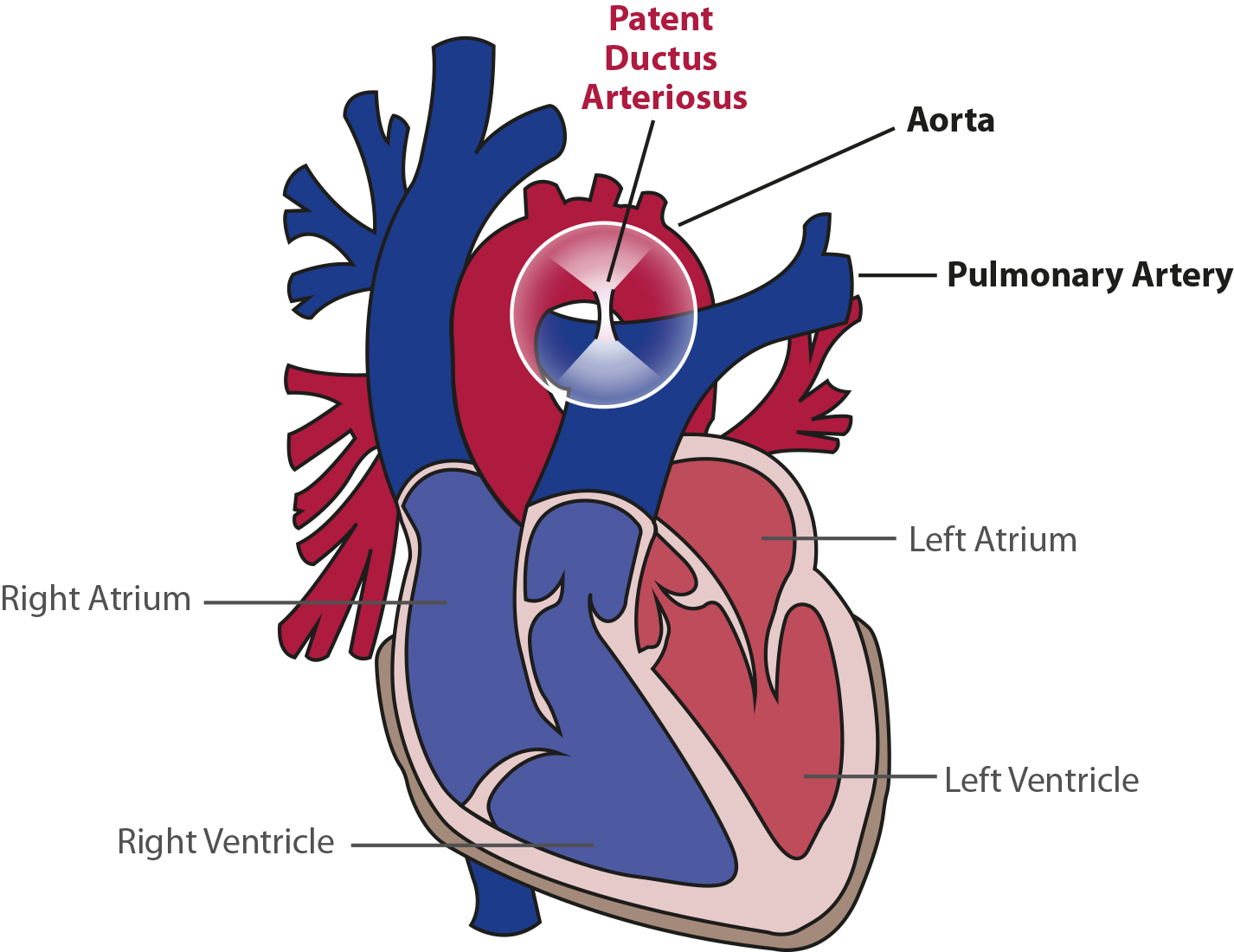
The ductus arteriosus is a natural blood vessel that connects the aorta (the body’s main artery, which carries oxygen-rich blood) to the pulmonary artery (which transports blood from the heart to the lungs for oxygenation) while the baby is still in the womb. This vessel allows blood to bypass the lungs before birth.
Normally, the ductus arteriosus closes shortly after birth. However, in about 60% of preterm infants, especially those born at the lowest gestational ages, this vessel remains open—a condition known as patent ductus arteriosus (PDA).
When the ductus arteriosus does not close, it can lead to serious complications involving the lungs, brain, and gastrointestinal system. Preterm babies with PDA are at a higher risk for health issues compared to those in whom the vessel closes naturally.
There are existing medications, such as indomethacin and ibuprofen, that can be used to close the ductus arteriosus. However, due to their potential adverse effects, these drugs are not recommended for prophylactic use—meaning they are not typically given before it's clear whether the ductus will close on its own.
Paracetamol (acetaminophen), a widely used medication in neonatal care, has recently shown promising results in closing the ductus arteriosus with fewer side effects. Commonly used for pain relief in newborns, paracetamol offers a safer alternative.
The TREOCAPA trial investigates whether prophylactic use of paracetamol in very preterm infants can effectively close the ductus arteriosus without causing harmful side effects.
The TREOCAPA study is divided into two phases: Phase II and Phase III.
| Phase II | Phase III | |
|---|---|---|
| Study Group | 30 preterm infants born at 23 to 26 weeks of gestation | 396 preterm infants born at 23 to 26 weeks; 398 preterm infants born at 27 to 28 weeks |
| Objective | Determine the minimum effective dose of paracetamol that can close the ductus arteriosus by day 7 after birth in preterm infants born between 23 and 28 weeks of gestation | Evaluate whether prophylactic use of paracetamol during the first five days of life is safe and effective in reducing the risk of death and severe complications of prematurity, such as brain hemorrhages (intraventricular bleeding), lung injuries, eye problems (e.g., Retinopathy of Prematurity). |
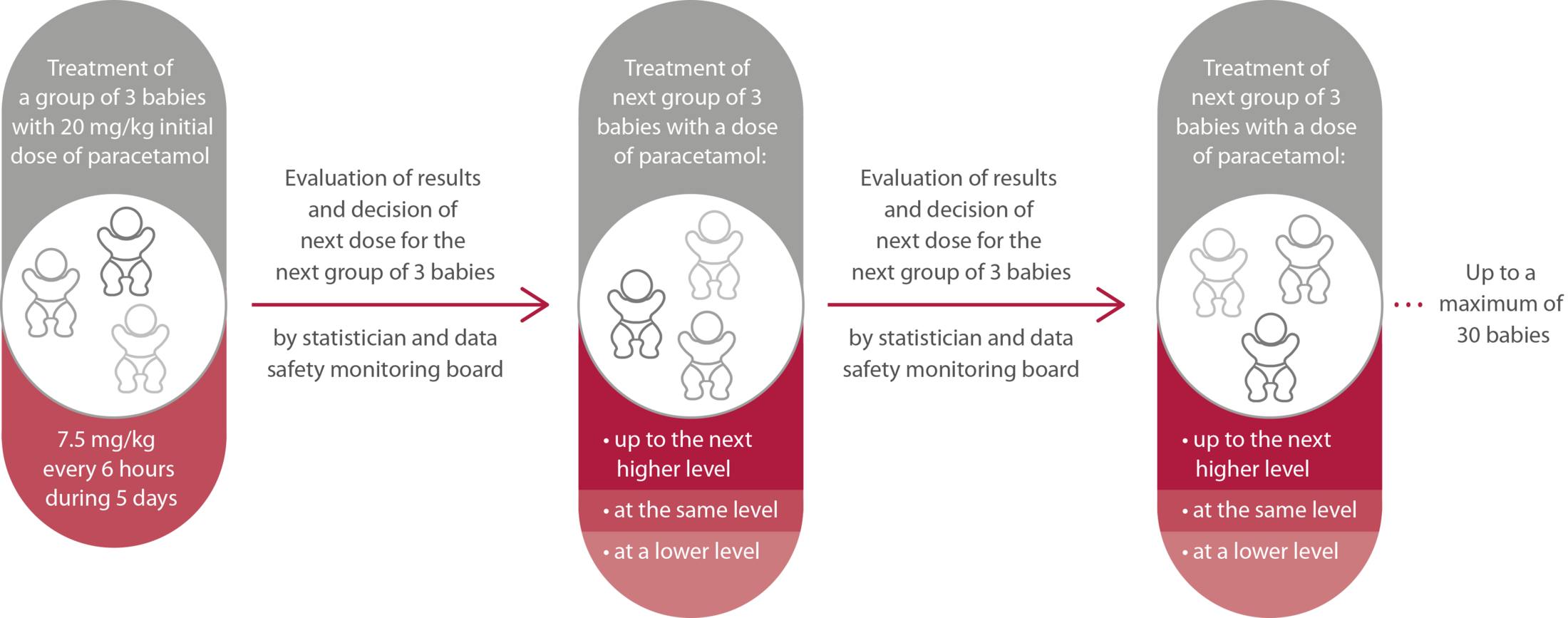
Figure 1. Study procedure in Phase II of the study.
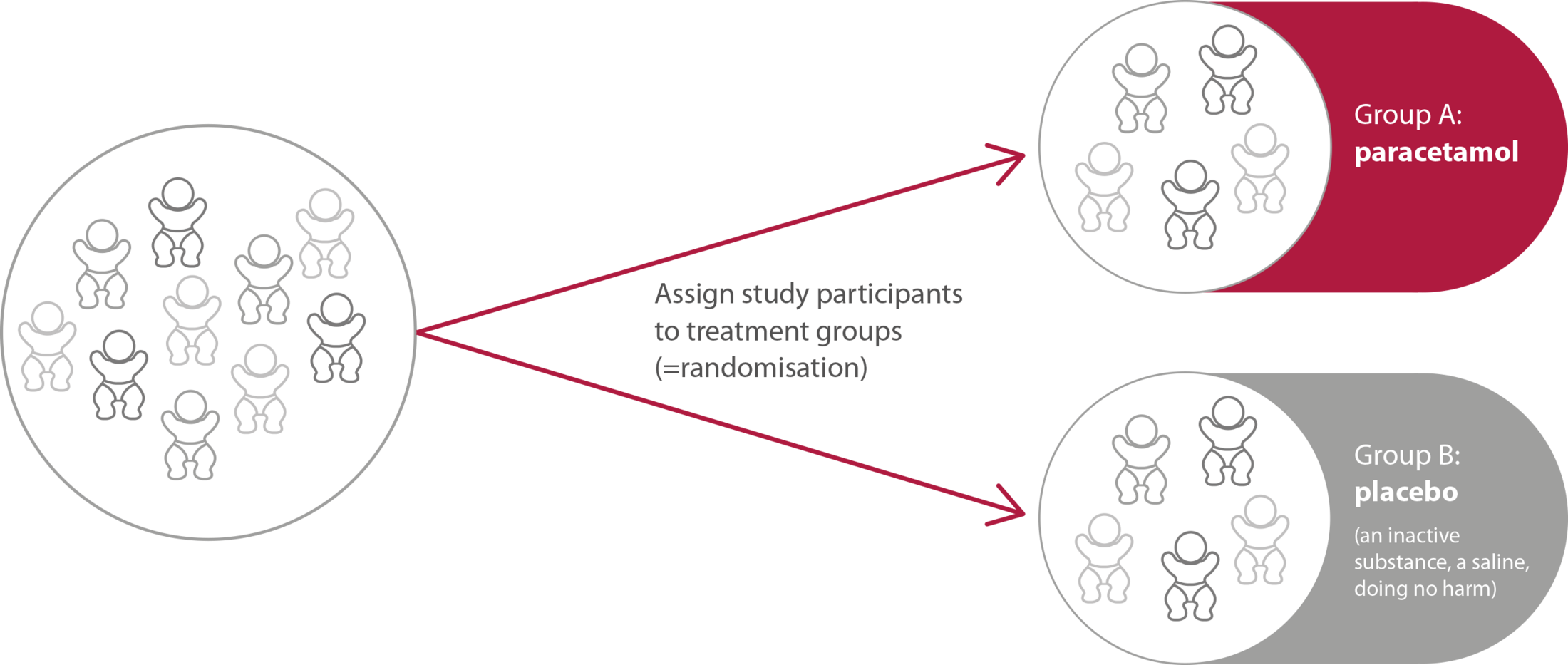
Figure 2. Allocation of the participants in the study group in Phase III.
GFCNI (formerly EFCNI) plays a vital role in ensuring public and patient involvement (PPI) throughout every phase of the TREOCAPA clinical trial. As part of this role, GFCNI coordinates and manages the Parental Advisory Board (PAB), providing a platform for parents of preterm infants to actively participate in the study.
Our goal is to ensure that parents are informed, supported, and empowered throughout the process—because the results of this research will directly impact the health and lives of their children.
In addition, GFCNI contributes to the development of trial materials and leads the project’s communication strategy, which is designed to:
Engage key stakeholders across the entire lifecycle of the study
Increase awareness and understanding of patent ductus arteriosus (PDA) and the potential of acetaminophen as a preventive treatment
Build greater support for the TREOCAPA trial and for neonatal clinical research more broadly
Ensure that the study results are clear, relevant, and accessible to all stakeholder groups
GFCNI works in close collaboration with the study team in France and other partners to implement these efforts through a variety of strategic activities.
INSERM, France
Nantes University Hospital, France
Even early interventions can have significant long-term effects on a child’s health and development. To capture these outcomes, the TREOCAPA-LT study was launched as a long-term follow-up to TREOCAPA.
This study investigates the neurological development of TREOCAPA participants at 24 months of age, offering a broader understanding of the lasting effects of prophylactic acetaminophen use in preterm infants.
What are the key differences between industry-sponsored and non-industry clinical trials? And what are the benefits of collaboration between the two? These questions aren’t always easy to answer at first glance.
This article provides a clear overview of both types of clinical trials and explores how collaboration enhances outcomes, with a special focus on the importance of the patient and parent voice in the research process.
Our latest factsheet offers a user-friendly overview of:
Key terms and definitions used in neonatal clinical research
The opportunities and challenges of conducting clinical trials in newborns
A practical breakdown of clinical trial design, using the TREOCAPA study as a real-world example
This resource is ideal for parents, healthcare professionals, and stakeholders looking to better understand how clinical trials work in the context of preterm and newborn care.
This three-part explanatory video series introduces you to the world of clinical trials, with a special focus on newborn and neonatal research.
In this series, you will learn:
What a clinical trial is and why it's important
Key terms and concepts commonly used in clinical research
The objectives and value of conducting clinical trials in newborns
What is it like to participate in a clinical trial with your newborn baby? In this powerful video, four families who participated in the TREOCAPA study share their honest reflections and insights.
The video covers:
Personal experiences of families involved in the TREOCAPA clinical trial
Whether parents would recommend participation in a neonatal trial to others—and why
The scientific importance of the TREOCAPA study, from the parents’ perspective
The next steps and follow-up actions that families hope to see once the trial concludes and results are published
We sincerely thank all the parents and families who shared their valuable experiences. Your voices are essential to improving neonatal care and advancing medical research in neonatology.

What do parents who have taken part in a clinical trial with their newborn baby wish for?
A family from Switzerland who participated in TREOCAPA shares their thoughts and emphasizes the importance of:
Preparing and presenting the study results in a clear and accessible way for a lay audience

What is important for parents when deciding to participate in a clinical trial with their newborn baby?
A family from France who joined the TREOCAPA study highlights the importance of:
Building a trust-based relationship between the parents and the study investigator

With the recruitment phase of the TREOCAPA study now complete, the entire study team would like to extend our heartfelt thanks to all the participants and their parents for their involvement and commitment.
Your valuable contribution made it possible to carry out this important research. We are deeply grateful for your time, trust, and engagement.
The test results collected during the trial will now be analyzed over the coming months and will contribute to new findings and scientific publications that advance care for preterm infants.
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No. 777389.
This Joint Undertaking is supported by the European Union’s Horizon 2020 research and innovation program and EFPIA (European Federation of Pharmaceutical Industries and Associations).
© 2025 GFCNI. All Rights Reserved.